High density of unrepaired genomic ribonucleotides leads to Topoisomerase 1-mediated severe growth defects in absence of ribonucleotide reductase
Tollervey lab paper featured in Nucleic Acids Research.
Authors
Cerritelli, S.M., Iranzo, J., Sharma, S., Chabes, A., Crouch, R.J., Tollervey, D. and El Hage, A.
Summary of Paper by Lori Koch
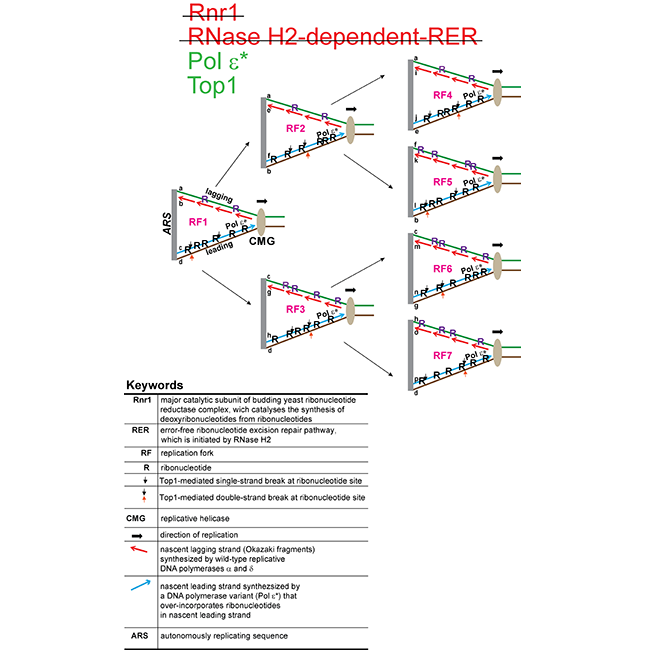
Every time a new cell is made, its DNA must be precisely copied. During DNA replication the key steps are unwinding of the double-stranded DNA into single strands, which then serve as templates to form two new DNA duplexes. Enzymes called DNA polymerases insert deoxyribonucleotides to build the new strands. To form deoxyribonucleotides, the ribonucleotide reductase (RNR) complex catalyzes the reduction of ribonucleotides into deoxyribonucleotides. In their recent study published in Nucleic Acids Research, Wellcome Centre scientists Aziz El Hage, David Tollervey, and colleagues revealed that depletion of Rnr1, the major catalytic subunit of the RNR complex in budding yeast, greatly increases the incorporation of ribonucleotides into genomic DNA. Initially, the authors discovered that cells lacking both Rnr1 and the ribonucleotide excision repair (RER) pathway grew more slowly than cells lacking only Rnr1. By quantifying the changes in DNA sensitivity to alkali treatment, they concluded that RNR and RER have a combinatory role in preventing the accumulation of genomic ribonucleotides. They then discovered that in the absence of RER and presence of a sub-optimal supply of deoxyribonucleotides, there is a threshold for toxic accumulation of genomic ribonucleotides. This is dependent on Topoisomerase 1-mediated DNA damage at sites of ribonucleotides. RNR inhibitors are widely used as chemotherapeutics, so these new data suggest that RNR inhibitors may be particularly effective in treating tumours that also carry mutations that weaken the RER pathway. Additionally, the authors’ work suggests that RNR may play a role in the human RNase H2-associated autoimmune disease, Aicardi-Goutières syndrome.

