Distinct roles of spindle checkpoint proteins in meiosis
Marston Lab – Current Biology
Authors
Mukherjee, A., Spanos, C. and Marston, A.L.
Summary of Paper by Cristina Cardenal Peralta, Proteomics Core, DRP-HCB
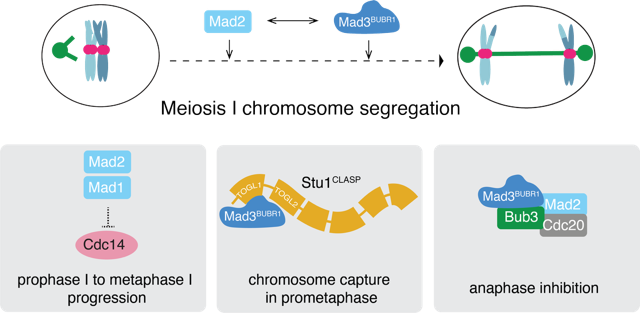
Meiosis, essential for gamete formation, involves a precisely coordinated sequence of events where homologous chromosomes undergo recombination and align at the cell’s equator before segregating to opposite poles. Errors in this process can result in infertility or birth defects. The spindle checkpoint acts as a quality control mechanism, halting cell cycle progression if chromosomes are not properly attached to microtubules. This checkpoint is primarily regulated by the mitotic checkpoint complex (MCC), which includes critical proteins like Mad2 and Mad3 (BUBR1).
In this study, researchers from the Marston lab discovered non-checkpoint roles for Mad2 and Mad3BUBR1 during meiosis I in budding yeast. They discovered that Mad2, but not Mad3, is crucial for the transition from prophase to metaphase I, ensuring the correct orientation of homologous chromosomes. To explore whether these differences were due to unique protein interactions, the team performed mass spectrometry analyses on immunoprecipitates from prophase/metaphase I stages.
These analyses revealed a novel interaction between Mad3 and Stu1CLASP, a microtubule-associated protein. Specifically, the TOGL1 domain of Stu1CLASP binds to Mad3, playing a key role in attaching chromosomes to the spindle. This interaction is vital for the segregation of homologous chromosomes that fail to properly biorient and for the accurate segregation of mini-chromosomes lacking crossovers.
Moreover, the simultaneous loss of Mad2 and Mad3BUBR1 results in a marked increase in chromosome missegregation, underscoring their complementary roles in maintaining meiotic fidelity. These findings uncover a previously unrecognized pathway involving Mad3BUBR1 and Stu1CLASP that promotes chromosome capture by microtubules, emphasizing the active role of spindle checkpoint proteins in ensuring precise chromosome alignment and segregation during meiosis I.

