A conserved CENP-E region mediates BubR1-independent recruitment to the outer corona at mitotic onset
Welburn Lab - Current Biology
Authors
Summary of Paper by Natalia Kochanova, Earnshaw Lab
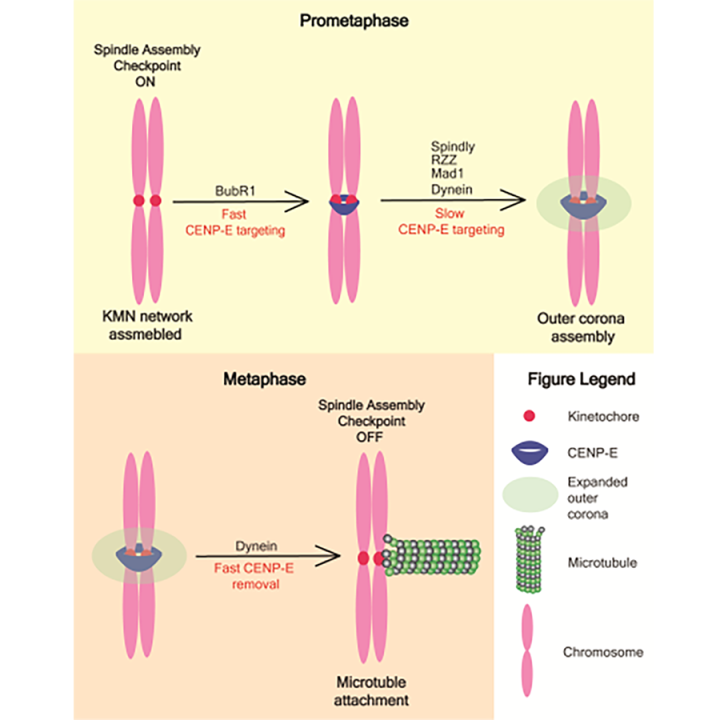
The Welburn lab discovered that CENP-E is recruited to kinetochores by two independent pathways. One recruitment mechanism is mediated by BubR1 protein, a component of the spindle assembly checkpoint (SAC), which prevents chromosomal segregation until all kinetochores are attached to microtubules. The other pathway includes the outer kinetochore corona and dynein, another motor protein important for cell division.
Indeed, depletion of BubR1, dynein heavy chain or both lead to a reduction of kinetochore CENP-E levels, having a synergistic effect. In agreement with this, a disruption of the RZZ complex, core component of the outer corona, also decreased CENP-E levels at kinetochores.
The team showed that the corona-targeting domain of CENP-E is distinct from the BubR1 targeting domain. The scientists also determined the X-ray structure of the part of this minimal corona-targeting domain and predicted BubR1-interacting domain structure by Alphafold. While the BubR1-interacting domain was predicted to be a four-helical bundle, the corona-targeting domain fragment was organized as a dimeric coiled-coil structure. Further modelling showed that the whole corona-targeting domain has a structure of two homodimeric coiled coils flanking a central conserved segment which is essential for its recruitment to the outer corona. The team then identified minimal requirement for CENP-E recruitment to unattached kinetochores in cells.
Overall, the lab carefully dissected two pathways contributing to the CENP-E recruitment to kinetochores, having shown that CENP-E requires structurally distinct domains for differing targeting.
Related Links

