The phospho-docking protein 14-3-3 regulates microtubule-associated proteins in oocytes including the chromosomal passenger Borealin
Ohkura lab paper featured in PLOS Genetics.
Authors
Repton, C., Cullen, C.F., Costa, M.F.A., Spanos, C., Rappsilber, J., and Ohkura, H.
Summary of Paper by Lori Koch
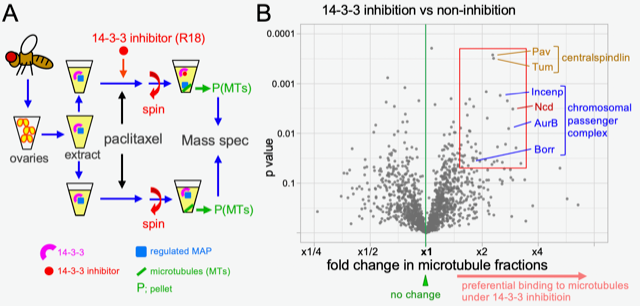
Sexual reproduction relies upon the accurate production of oocyte and sperm cells containing half the genetic information stored in DNA and protein that make up structures called chromosomes. The transmission of chromosomes into mature oocytes or sperm relies upon a structure called the spindle composed of fibres called microtubules. However, in many organisms including humans and fruit flies, the oocyte spindle only takes up a small overall fraction of the cell volume. Research suggests that chromosomes emit signals which promote spindle assembly. In their recent study published in PLOS Genetics, Dr. Charlotte Repton and her colleagues in Hiro Ohkura’s lab present evidence that spatial regulation of the protein 14-3-3 promotes the activity of the Chromosomal Passenger Complex (CPC) in the vicinity of chromosomes. Previous work in the lab identified that 14-3-3 regulates the microtubule motor kinesin-14/Ncd. To identify other spindle proteins regulated by 14-3-3, they isolated all proteins associated with microtubules from ovaries either untreated or treated with a chemical inhibitor of 14-3-3 and quantified their abundance by mass spectrometry. This quantified 1500 proteins and identified that 67 had highly different levels between the two treatment conditions. Interestingly, several members of the Chromosomal Passenger Complex (CPC) were significantly enriched on microtubules when 14-3-3 was inhibited. The CPC promotes proper orientation of chromosomes on the spindle. Interestingly, they identified that the CPC protein Borealin contains a predicted 14-3-3 binding motif. In vitro experiments demonstrated that Borealin binds to 14-3-3 directly and this depends on phosphorylation of Borealin S163. They noticed that a nearby residue, S161, matches the motif recognized by the CPC kinase Aurora B. Further in vitro experiments showed that Aurora B phosphorylation reduced Borealin binding to 14-3-3. Finally, they demonstrated that in living fruit flies, if Borealin is not phosphorylated at S163 it does not localize to spindles or chromosomes, confirming its importance. Overall they propose that 14-3-3 binding to Borealin prevents its binding to microtubules but that Aurora B phosphorylation of Borealin in the vicinity of chromosomes reverses this, allowing CPC to bind microtubules and promote the proper microtubule-chromosome attachment

