Rrp5 binding at multiple sites coordinates pre-rRNA processing and assembly
Tollervey lab paper featured in Molecular Cell.
Authors
Lebaron, S., Segerstolpe, A., French, S.L., Dudnakova, T., de lima Alves, F Granneman, S., Rappsilber, J., Beyer, A.L., Wieslander, L. and Tollervey, D.
Summary
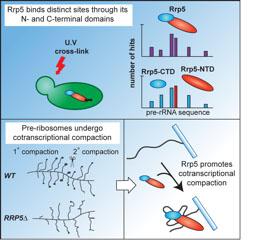
Direct RNA and protein interaction partners were identified for the highly conserved, multidomain ribosome synthesis factor Rrp5. Multiple, dispersed Rrp5-binding sites were identified in the pre-rRNA, with the N-terminal and C-terminal domains of Rrp5 each binding adjacent to RNA cleavage sites for which they are required. Rrp5 is required for pre-ribosome compaction and directly binds to large, structural proteins and NTPases. These potentially form a structural framework to coordinate large scale RNP folding during ribosome biogenesis.

