Histone divergence in trypanosomes results in unique alterations to nucleosome structure
Wilson Lab - Nucleic Acids Research
Authors
Deák, G., Wapenaar, H., Sandoval, G., Chen, R., Taylor, M.R.D., Burdett, H., Watson, J.A., Tuijtel, M.W., Webb, S. and Wilson, M.D.
Summary of Paper by Melanie Lim
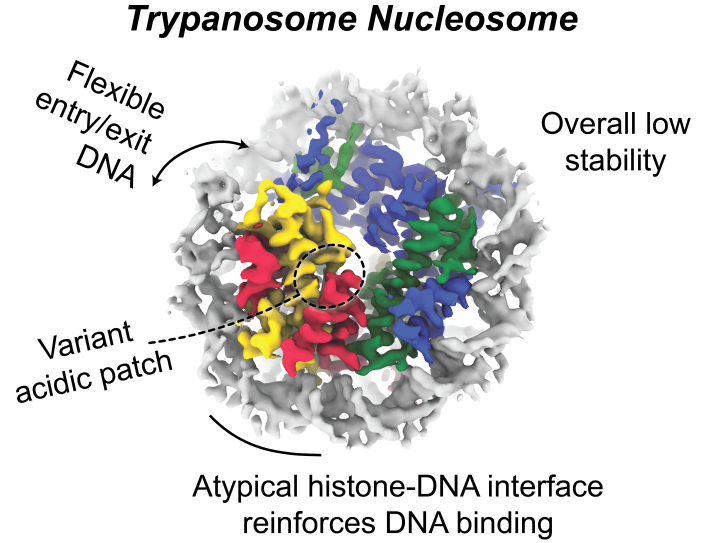
Imagine having to stretch out our cell’s DNA, the information-encoding material necessary for cellular function – it would approximately traverse the height of a door. One way to deal with this immense amount of genetic material is to wrap DNA around two sets of a four-protein complex called histones. A single histone-DNA unit is known as a nucleosome core particle (NCP). This genome organisation method is shared from yeasts to humans with histone sequences being highly conserved. However, subtle changes can affect DNA interaction, consequently impacting the structure and function of NCPs. In a recent paper published in Nucleic Acids Research, Deák and colleagues from the Wilson lab set out to elucidate the structure of the NCP from a highly divergent parasite Trypanosoma brucei (T. brucei), the causative agent of trypanosomiasis. Unusually histones are highly different in this species. They used cryo-electron microscopy to build a high-resolution structural model of the T. brucei NCP. With this model and other biochemical techniques, the authors concluded that while overall architecture were largely similar to those found in humans, the T. brucei NCP is both less stable and less compact. The authors reasoned that in T. brucei histones, amino acid substitutions alter the interactions between the components of the histone molecule and those between histone and DNA. Observations from the NCP model and binding assays also found that the acidic patch, a well-characterised negatively charged surface on histones, has a different binding property when compared to human histones. This hints towards a potential drug target that is selective for T. brucei. Moving forward, this NCP model will help us further understand the details of T. brucei histone-DNA binding and could aid future drug discovery to treat trypanosomiasis.

